![]()
Research
People
Profiles
How to Reach Us
Contact Info
Harvey Lodish
Harvey was born in Cleveland, Ohio on November 16, 1941. His father Nathan was a manager at a small company manufacturing electrical fittings for houses and autos. His mother Sylvia was a teacher before her marriage; after her children were in school she earned her BA degree and taught 3rd grade in Cleveland Heights. Harvey's first publication was in 1946, a letter published in a children's' column in the Cleveland Press. Harvey started at Chesterfield Elementary School in Cleveland but transferred to Taylor Elementary School in Cleveland Heights when his family (in fact his entire neighborhood) moved to the suburbs in 1951. At Taylor Harvey was appointed head of the school crossing guards, giving him authority over about 20 5th and 6thgrade students.
After three years at Roosevelt Junior High
Harvey entered Cleveland Heights High School, where he distinguished himself
keeping time as the bass drummer in a 164- piece marching band. He also
participated in a small research project — measuring the levels of
various nitrogenous compounds in the blood — directed by Dr. Ethel
Laughlin, a chemistry teacher. During the summers of 1958, ’59, and
’60 Harvey worked at Western Reserve (now Case Western Reserve) Medical
School with Dr. Robert Eckel studying potassium transport in red blood
cells. This led to his first scientific publication in 1960, and he has
been studying red blood cells ever since. (And in 1982 he was elected
to the Cleveland Heights High School Alumni Hall of Fame, an honor he
shares with his two younger brothers Leonard and Richard).
Harvey entered Kenyon College in 1959 and graduated three years later, summa cum laude and with Highest Honors in Chemistry and Highest Honors in Mathematics. During the summer of 1961 he worked at Stanford in the chemistry laboratory of Dr. Carl Djerassi, a Kenyon alumnus and “discoverer of the birth control pill.” He saw first hand how close contacts between universities and companies (Syntex in this case) can be mutually profitable and result in excellent science. Harvey was awarded an honorary D. Sc. degree from Kenyon in 1982 and from 1989 to 2007 served as a member of the Kenyon College Board of Trustees. From 2003 - 2007 he was also a member of the Board Executive Committee.
Harvey received his Ph.D. degree in genetics with Dr. Norton Zinder from the Rockefeller University in 1966; his thesis focused on a genetic and biochemical analysis of the three genes in the RNA bacteriophage f2. Then followed two years of postdoctoral research at the M.R.C. Laboratory of Molecular Biology with Drs. Sydney Brenner and Francis Crick; there he determined the factors that control initiation of translation of the three f2 genes. He joined the faculty of the MIT Department of Biology in 1968. Harvey was promoted to Professor in 1976, and in 1983 was appointed a Founding Member of the new Whitehead Institute for Biomedical Research. In 1999 he also became Professor of Bioengineering in the new MIT Department of Bioengineering. His one “real” sabbatical was in 1977- 78 as a Guggenheim Fellow at the Imperial Cancer Research Fund in London with Robin Weiss.
Initially, his work at MIT focused on translational control of protein synthesis, collaborating with David Baltimore's lab on translation of poliovirus mRNA. David Housman, one of his first Ph.D. students, showed that all mammalian proteins begin with a methionine residue transferred from a specific met-initiator tRNA.
From 1971 through 1987 many in his laboratory studied the regulation of gene expression during differentiation of the cellular slime mold Dictyostelium discoideum, focusing on the details of mRNA biogenesis and on identifying proteins and mRNAs expressed at specific times of differentiation and in either prespore or prestalk cells.
Beginning in 1973, his laboratory has concentrated on the biogenesis, structure, and function of several important secreted and plasma membrane glycoproteins. Working in part with the Baltimore lab his group defined the biosynthesis and maturation of the vesicular stomatitis virus envelope glycoprotein, elucidating the now well-known ER → Golgi → plasma membrane pathway for biogenesis of cell surface proteins. With Blobel they developed the first in vitro system for studying the biosynthesis, membrane insertion, and glycosylation of an integral membrane protein, and then demonstrated that removal of the signal sequence and addition of the two N-linked carbohydrates occurs cotranslationally. Later work identified the intracellular organelles that mediate recycling of the asialoglycoprotein and transferrin receptors, and clarified the role of pH changes in delivery of iron to cells and recycling of the transferrin receptor. His group also elucidated steps in folding and oligomerization of several proteins within the endoplasmic reticulum, showed that exit of several newly-made secreted proteins from this organelle requires that they be properly folded, and developed probes for measurement of the redox state within the endoplasmic reticulum.
His group was the first to clone and sequence mRNAs
encoding a mammalian glucose transport protein (the
red cell glucose transporter, GLUT1), and then they identified
and worked out the roles of a family of glucose transporters, including
GLUT2 and GLUT4, in liver, muscle, and adipose tissue. After cloning Band
III, the red cell anion exchange protein, his group elucidated the role
of this protein and its homologs in acid-base balance in the kidney.
Other proteins cloned by members of his lab include the first transporter
for free fatty acids, the two subunits of the hepatic asialoglycoprotein
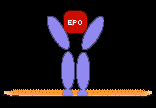
receptor, intestinal sucrose-isomaltase, the erythropoietin receptor,
the calcitonin and endothelin receptors, and (with the Weinberg lab) two
subunits of the TGFß receptor. These have been used to define the
structure and biosynthesis of these and related proteins and to identify
and characterize related genes that encode proteins of related physiological
function.
For example, cloning of the erythropoietin receptor made possible a long series of studies on the signal transduction pathways it activates and the mRNAs induced and repressed at several stages of erythropoiesis, leading to an understanding of how it prevents apoptosis of erythroid progenitor cells and allows them to undergo a process of terminal proliferation and erythroid differentiation. Cloning of the TGFß receptors led to elucidation of the role of the Smad signal transduction proteins in activation of transcription of genes encoding extracellular matrix proteins and inhibitors of the cell cycle, and to an understanding of how loss of these receptors contributes to tumor development. Later work led to the cloning and characterization of other factors in the TGFß signaling pathway including, with the Weinberg lab, several oncogenes that block TGFß signaling pathways.
Besides studying the erythropoietin and TGFß receptors and the family of mammalian fatty acid transport proteins they cloned, his lab has been studying the insulin receptor and determining how it signals fat cells to increase uptake of both sugars and fatty acids. His group also investigates the receptors and mechanism of action of adiponectin, a new adipocyte-produced hormone they cloned that potently enhances fat and glucose metabolism by muscle, inhibits gluconeogenesis by liver, and causes prolonged weight loss in mice.
In the past decade microRNAs have emerged as important posttranslational regulators of gene expression. His group defined roles of many miRNAs in differentiation of erythroid and lymphoid progenitor cells and in several hematopoietic cancers, and in regulating adipocyte and muscle differentiation and function.
Finally his group studies hematopoietic stem cells. His group has identified several novel growth factors that, added together, allow expansion of both mouse bone marrow and human cord blood hematopoietic stem cells greater than 20 fold. They are working with colleagues to apply this system to clinical transplants where stem cells are often in short supply. His lab has also identified new functional cell surface markers for these cells, including endoglin and (together with the Lindquist lab) the normal prion protein.
On a personal level, Harvey married Pamela Chentow in 1963, and they have three married children - Heidi (born in 1966 and a social worker), Martin (1968, a high school physics and chemistry teacher and administrator) and Stephanie (1969, a pediatrician). They have seven adorable grandchildren — Sophie (1997), Joshua (1998), and Tobias (2005) to Martin and Kristin; Emma (1999) and Andrew (2002) to Heidi and Eric Steinert, and Isaac (2000) and Violet (2004) to Stephanie and Bruce Peabody. Harvey is a member of the Appalachian Mountain Club 4000—foot club, having climbed on foot all 68 New England peaks over 4000 ft. high. He enjoys working out with his trainer; reading, writing, thinking, and talking; traveling with his wife Pam to rural areas of Southeast Asia; hiking with his children and students; and playing with his grandchildren.
Harvey was on the Editorial Board of the Proceedings of the National Academy of Sciences. He was on the Board of Reviewing Editors of Science and was Editor of Molecular and Cellular Biology from 1981 to 1987. Harvey has been on the editorial boards of a number of other journals, including the Journal of Cell Biology, the Journal of Biological Chemistry, and Nucleic Acids Research. He has served on advisory panels for the NIH, NSF, and American Cancer Society, and on the advisory boards of several institutions, including the Biozentrum of the University of Basle, the European Molecular Biology Laboratory in Heidelberg, the Center for Molecular Biology Heidelberg (ZMBH), the Life Sciences Institute of the University of Michigan, and the PEW Scholars Program in Biomedical Sciences. He chaired the advisory boards of the Division of Basic Sciences of the Fred Hutchinson Cancer Center and the Cleveland Clinic Lerner Research Institute.
Currently he is a member of the advisory board of the California Institute of Technology Division of Biology and of the Engineering Division of the University of California Santa Barbara.
Since 2006 he has served as a member of the Board of Trustees of Children's Hospital Boston, where he also serves as Chair the Board of Trustees Research Committee. He is also Founding Chair of the Scientific Advisory Board of the Massachusetts Life Sciences Center, the group charged with oversight of the state's 10-year $1 billion investment in the life sciences.
During the 2004 calendar year Dr. Lodish served as President of the American Society for Cell Biology.
Harvey has been the lead author of the textbook Molecular Cell Biology, now in its sixth edition and translated into six languages. Together with six co-authors he is currently writing the 7th edition, due in 2011.
He was elected a Member of the National Academy of Sciences (1987), a Fellow of the American Association for the Advancement of Science (1986), a Fellow of the American Academy of Arts and Sciences (1999), and a Fellow of the American Academy of Microbiology (1992). He is also an Associate (Foreign) Member of the European Molecular Biology Organization (1996). Dr. Lodish received a MERIT award from the National Institute of Diabetes and Digestive and Kidney Diseases. He is also a recipient of the Stadie Award from the American Diabetes Association and is listed in Who's Who in America (1987).
Harvey has been very active in the biotechnology industry. He was a founder and scientific advisory board member of Genzyme, Inc., Arris Pharmaceuticals, Inc, Millennium Pharmaceuticals, Inc, and Allozyne, Inc. He previously served on the Scientific Advisory Boards of the Eisai Research Institute, AstraZeneca Pharmaceuticals, Genset SA, and Dyax Corporations.